Measurement of non-photochemical quenching using the JTS-150 pump probe spectrometerPhotosynthesis – Application Note 5
Latest updated: June 13, 2024Introduction
Here we describe how to measure Non- Photochemical Quenching (NPQ) using the JTS-150. This method has been long utilized by the previous models of JTS and provides data that is analogous to a pulsed-amplitude modulated (PAM) fluorometer. PAM fluorometers have been powerful tools that were based on the model that absorbed solar radiation has 3 possible fates, to be released as heat, fluorescence, or drive photochemistry. The photochemical pathway leads to a quenching of observed fluorescence that is therefore referred to as photo- chemical quenching.
Chlorophyll fluorescence is a widely used technique and has been the subject of several excellent reviews that we would recommend before starting experiments if the techniques are unfamiliar, please see [1, 2, 3]. Separation of fluorescence quenching into photo- chemical and non-photochemical components was first achieved by the addition of 3-(3,4- dichlorophenyl)-1,1-dimethylurea (DCMU) to intact chloroplasts and Chlorella cells at points throughout the fluorescence induction curve. DCMU inhibits electron transfer from QA to the secondary quinone acceptor of PSII (QB), which results in a rapid reduction of QA and an in- crease in fluorescence as photochemical quenching is prevented. The development of PAM flu- orometers that use weak modulated measuring beams in which phase and frequency decoding are used to detect fluorescence yield changes enabled the routine, nondestructive, quantitative determination of photochemical and non- photochemical processes in leaves.
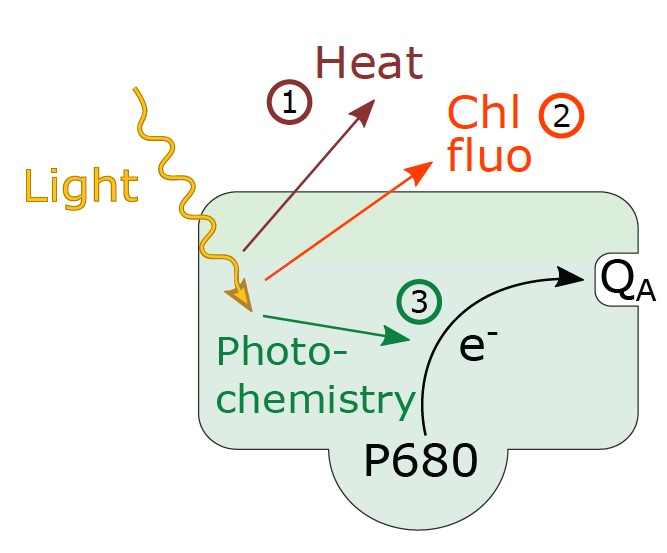
Figure 1: Light energy absorbed by chlorophylls associated with PSII can be used to drive photo- chemistry in which an electron (e−) is transferred from the reaction center chlorophyll, P680, to the primary quinone acceptor of PSII, QA. Alternatively, absorbed light energy can be lost from PSII as chlorophyll fluorescence or heat. The processes of photochemistry, chlorophyll fluorescence, and heat loss are in direct competition for excitation energy, so if one rate increases the other will decrease.
The use of DCMU is replaced by application of brief (less than 1 s) saturating flashes of light sufficiently intense as to maximally reduce the QA pool in the sample. This technique was used to demonstrate that the quantum yield of PSII photochemistry of a leaf at a given actinic light intensity can be estimated from the modulated fluorescence yield prior to the application of the saturating flash and the maximum modulated fluorescence yield during the flash.
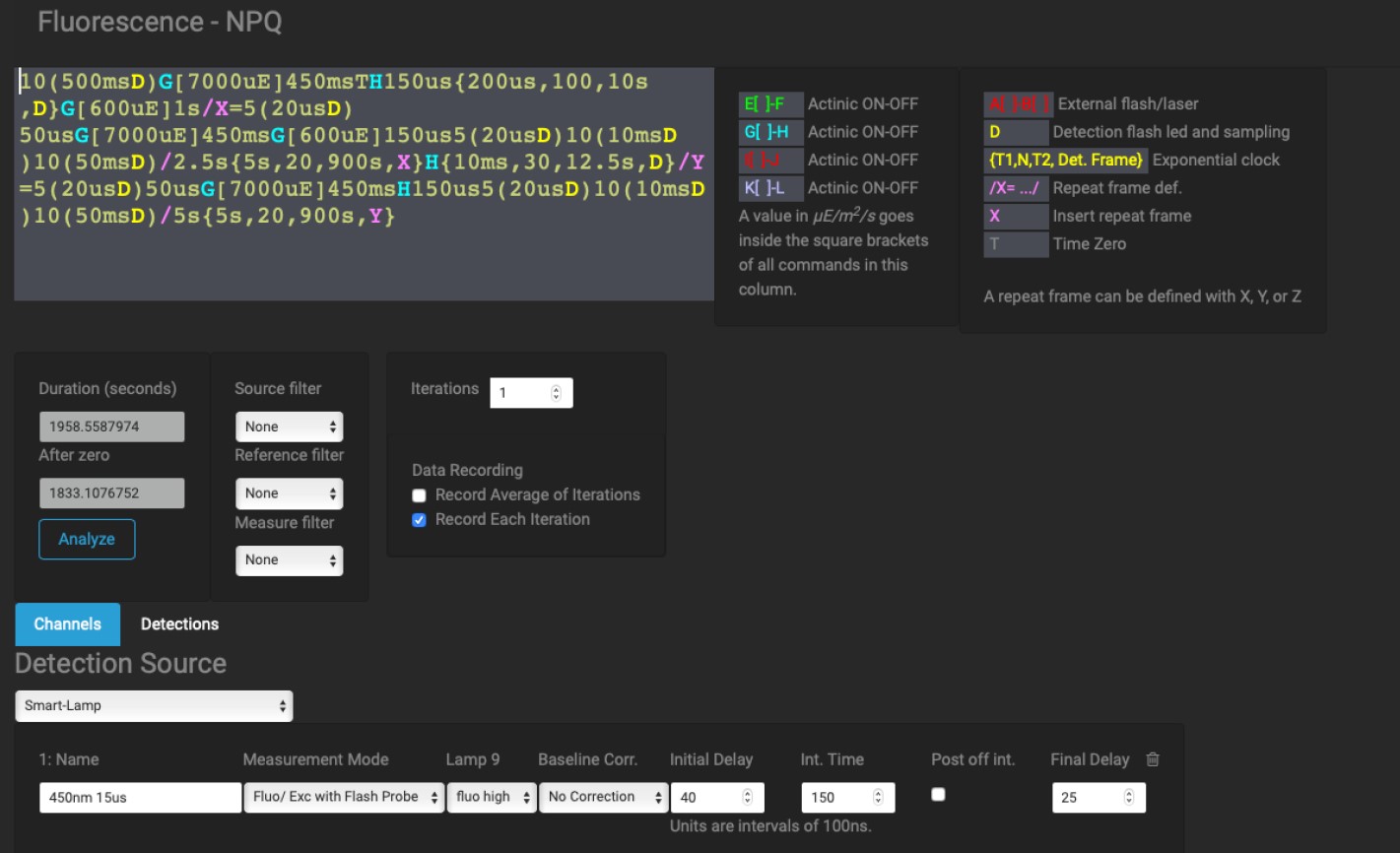
Figure 2: Screenshot of example NPQ sequence from the sequence page of Photo-Kine2.
The implementation of this model in the con- text of the JTS is slightly different since there is no modulation of the measuring beam coupled to phase decoding as in a PAM fluorometer. The JTS rather has exquisite control in the time domain of all the LEDs utilized in the experiment. In this case, we will be applying saturating flashes to temporarily induce complete reduction of the QA pool, but we utilize a brief flash probe that is analogous to the modulated measuring beam just after the saturating flash when the actinic lamp has returned to the non- saturating actinic value defined in the sequence. It is critical to understand that the flash probe is for use in the light at the actinic level, not during the saturating flash. There is a period after the flash of several hundred microseconds that the same value will be obtained. Since the JTS does not use a modulated measuring beam, there is not a possibility of measuring the relative fluorescence changes during the saturating flash. However, the flash probe will reveal the exact same change in fluorescence yield observed by the PAM fluorometer and allow the calculation of all the same parameters seen in [1].
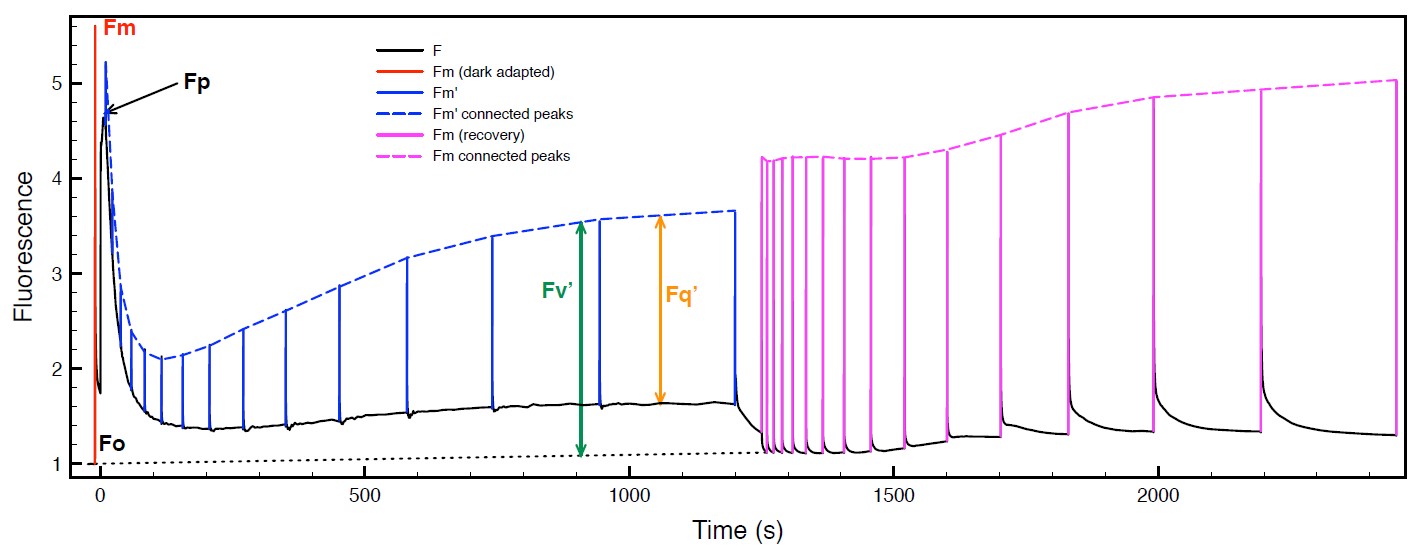
Please refer to figure 6 for a graphical representation of the raw data parameters and figure 7 for the derived data summarized in Table 1 on the following page.
Method
Optical Bench Setup First the optical bench must be setup for the measurement. Place the BG-39 filter in the reference detector to keep stray actinic light out, then place the Fluo filter in the measure detector with absorbance portion of the filter (the dark red side rather than the mirrored side) facing the sample. Place a relevant sample either in the leaf or cuvette holder and push the sample and detector as close as possible to the output of the optical bench and lock in place using the thumb screw of the measure detector. Place a detection lamp with fluo flash probe blue LEDs, (the Smart-Lamp in this example), and balance the Ref. and Meas. detectors at around 0.03–0.05 V.
Table 1: Frequently used chlorophyll fluorescence parameters and their mathematical expressions, please see figure 6 and figure 7 on page 8 for further details.
| Parameter | Description | Relevance to Physiology |
|---|---|---|
| F, F’ | Fluorescence emission from dark (w/o prime) or light(w/ prime) adapted sample | Does not provide insight on PS without additional factors (see below) |
| Fo, Fo’ | Minimal fluo. from dark (w/o prime) or light (w/prime) adapted sample | Fluo. level corresponding to QA maximally oxidized i.e. all PSII reaction centers “open” |
| Fm, Fm’ | Maximum fluo. from dark(w/o prime) or light (w/prime) adapted sample | Fluo. level corresponding to QA maximally reduced i.e. all PSII reaction centers “closed” |
| Fv, Fv’ | Variable fluo. from dark(w/o prime) or light (w/prime) adapted sample | Ability of PSII to photochemically reduce QA |
| Fq’ | F ′m − F ′, Quenched fluo. or difference between Fm’ and F’ | Photochemical fluo. quenching by open PSII |
| Fq’ / Fm’ | PSII operating efficiency | Also referred to as ϕPSII, this quantity is the efficiency of QA reduction by absorbed light. This is a good estimate for Linear Electron Flux. |
| Fv / Fm | Max. quantum efficiency of PSII | Max. efficiency that light absorbed by PSII can reduce QA. |
| Fv’ / Fm’ | Max. PSII efficiency | Max. efficiency of PSII photochemistry at a given PPFD. |
| Fq’ / Fv’ | PSII efficiency factor | Relates Fv′/Fm′ to Fq′/Fm′ and is nonlinearly related to “open” PSII reaction centers. Also termed qP. |
| NPQ | Nonphotochemical quenching | Apparent rate of heat loss from PSII, and is given by: (Fm/Fm′) − 1 |
Figure 3: Use the Data Process menu to analyze the fluorescence results. The toolbar at the bottom of the Analysis page in Photo-Kine 2 contains a tool for processing fluorescence data.
Sequence The example sequence in this note has 3 sections: 1) Baseline and determination of Fm, 2) Induction of NPQ during actinic period, at 600 mE m-2 s-1 in this example, and 3) Dark recovery. We will discuss each section of the sequence in detail.
Section 1 begins with a dark baseline from the portion 10(100msD). This is the equivalent of the Fo value as measured with a PAM fluorometer. The next part of the sequence performs a saturating flash at 7000 mE m-2 s-1 for 450 ms and is followed by an exponential clock detection scheme. The rationale for the exponential clock is to take data rapidly while rapid changes are occurring and then to space out the detections further and further as the changes are slower in the later portion of the decay. This gives a good representation of the recovery without much perturbation from actinic effects of the detection lamp. This effect could also be achieved by manually placing the detections.
The next section of the sequence is the actinic portion, during this phase of the experiment, the actinic light is consistently on at 600 mE m-2 s-1 for a total of 900 s plus the sum of the detection time or about 9.45 s. The actinic lamp is set by G[600uE] 1s for 1s before the first detection, which in the example, is also defined as the X detection frame /X=5(20usD)50usG[7000uE]450msG[150uE]150 us5(20usD)/. Using the X detection frame al- lows this entire action to occur repeatedly using only X in the sequence to represent the entire saturating flash detection. The detection frame uses 5 rapid detection flashes separated by 20 ms to measure F’ and then applies 7000 mE m-2 s-1 for 450 ms followed by setting the actinic source back to 600 mE m-2 s-1. After the saturating pulse, another set of 5 rapid detections occur measuring Fm’.
The dark recovery is similar to the actinic period in structure but turns the actinic source off between saturating pulses used to measure Fm during the recovery. First we monitor the decay of Fo’ with H{10ms,30,12.5s,D} for 12.5 s. Again we utilize the detection frame, this time Y is defined by /Y=5(20usD)50usG[7000uE]450msH150us5(20 usD)10(10msD)10(50msD)/5s. Once Y is defined, we create an exponential clock similar to the actinic period with {5s,20,900s,Y}.
- Section 1, Baseline and saturating pulse for Fo and Fm:
10(500msD) G[7000uE] 450ms T H 150us {200us, 100, 10s, D} - Section 2, Actinic period with F’ and saturating pulses for Fm’ :
G[600uE] 1s /X= 5(20usD) 50us G[7000uE] 450ms G[600uE] 150us 5(20usD) 10(10msD) 10(50msD)/ 2.5s {5s, 20, 900s, X} - Section 3, Dark recovery with Fo’ and saturating pulses for Fm:
H {10ms, 30, 12.5s, D} /Y= 5(20usD) 50us G[7000uE] 450ms H 150us 5(20usD) 10(10msD) 10(50msD)/ 5s {5s, 20, 900s, Y}
Sample concerns In a typical NPQ experiment, the leaves should be dark adapted for at least 20 minutes. Ideally, there would also be a controlled gas mixture or at least ambient air pumped across the backside of leaf samples. In the example data, the sample was a 10 mm diameter leaf disc from a mature leaf of a crape myrtle (Lagerstroemia indica) bush with no air pumping. For algae and cyanobacteria, a chlorophyll concentration of 5-30 mg/ml is a good starting value. If there are significant amounts of phycobilisomes present, then you will need to determine a good working concentration by other methods.
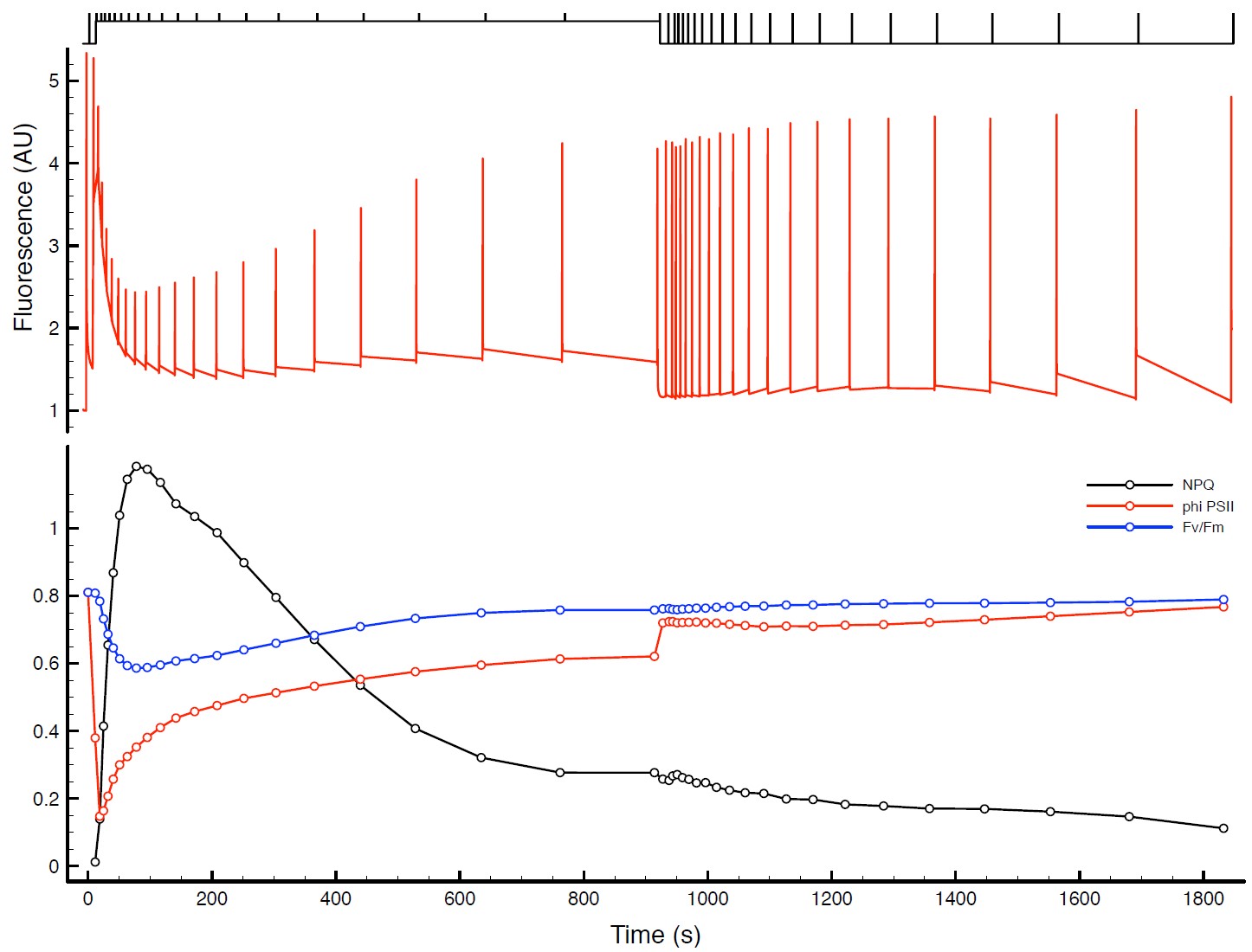
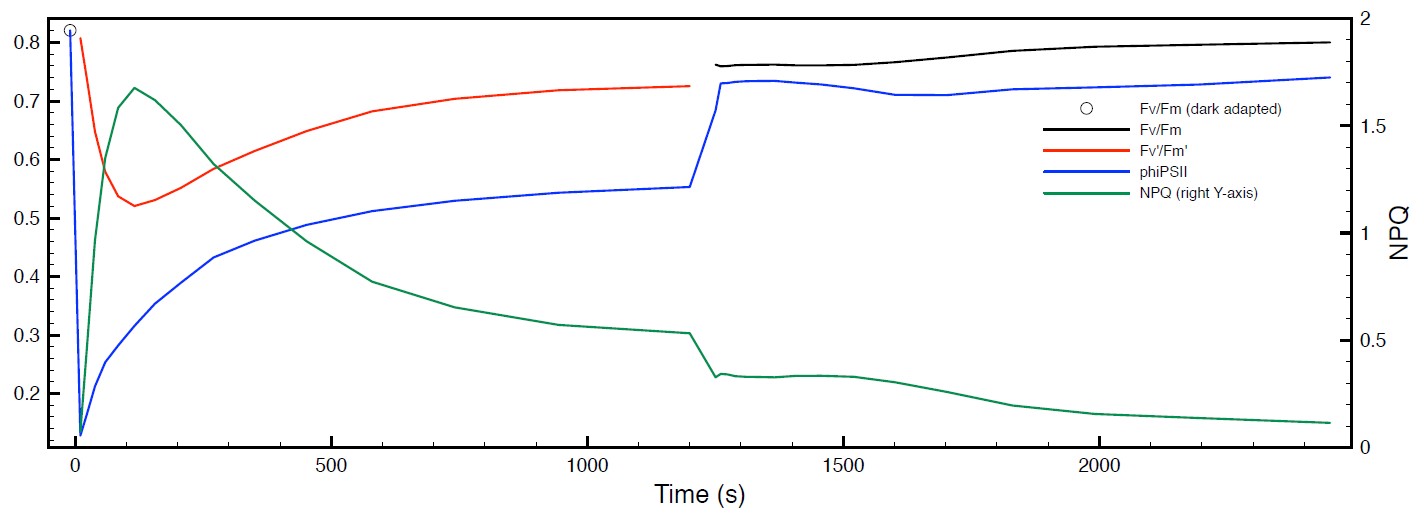
Figure 4: Raw data from example NPQ experiment. The fluorescence data are shown in the upper panel with a representation of the actinic light source from the sequence shown above in black. The saturating pulses are 7000 mE m-2 s-1 and the actinic light on period is at 600 mE m-2 s-1. The lower panel depicts the resulting NPQ (black trace), ϕPSII (red trace) and Fv /Fm (blue trace) values.
Analyzing the data Once acceptable data have been collected, begin the analysis from the Analysis page of Photo-Kine 2. The toolbar depicted in 3 on page 3 is found below the plot. Click the calculator icon for the data process menu. The user input for analyzing NPQ data are seen in 5 on the following page. The NPQ analysis is the third tab within the Data Process dialog, and allows you to indicate which fluorescence parameters you would like to calculate by checking the appropriate box for each.
In order to perform the analysis, the algorithm must identify the saturating flashes and which points you would like to use for the calculation of F or F’ and Fm or Fm’. The points included in the average for Fm may be chosen either by a fixed number of points before and after the flash, or using a time window before and after the flash. The threshold for a saturating pulse must be a value greater than the actinic value and less than the saturation pulse. In the context of the example, any value between 601–6999 mE m-2 s-1would give the same result when serving as the threshold, the most generalizable choice is to use saturation intensity minus 1. There is no restriction on which lamp is used, so the user must also indicate which lamp is to be considered providing the saturation pulse. The results can also be added to any data groups that you have already prepared before clicking the Data Process menu.
Figure 5: Screenshot of PhotoKine2 fluorescence analysis tool. Desired parameters are chosen by the user and the method of identifying saturating pulses form the sequence is indicated by the user. Input data and output into groups is achieved in the data browser on the right side of the menu.
Results
The raw data from the example sequence is dis- played in Figure 4 on the previous page in the upper panel. When the balance is ideal, the Fo value will be very near 1 and in healthy leaves, the Fv / Fm value is remarkably consistent in the range of 0.81-0.84, so when the balance is nearly ideal, the Fm value is typically around 5.25. The F’ values rise rapidly and then begin to fall and finally approach steady state values at around 300 s in this example, however the Fv’ values continue to change until about 800 s have elapsed in the actinic period. Typical durations of the actinic and recovery periods are on the order of 15-25 minutes. In the example data, we have barely achieved steady state values of the derived data in the lower panel from 800-900 s, and that the recovery is incomplete. If these are critical parameters for your work, please in- crease the duration of these periods. If using exponential clocks as in the example, you may just change the duration parameter in the actinic and recovery phases.
The ϕPSII value drops rapidly in the dark to light transition indicating that most of the QA is reduced very rapidly at this level of illumination. As the plant responds to the light level, the PSII operating efficiency improves. The NPQ value also rapidly rises form near zero to a maximal value in about 100 s.
References
- Neil Baker. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annual Review of Plant Biology, 59(1):89–113, June 2008.
- Kate Maxwell and Giles N. Johnson. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany, 51(345):659–668, April 2000.
- H. Murchie and T. Lawson. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. Journal of Experimental Botany, 64(13):3983–3998, October 2013.
Fluorescence Parameters Definitions
This section has been included as a reference to the definitions of each of the fluorescence parameters described in [1]. As a clarification, these data were collected on a JTS-150, but not with the sequence above, see figure 2 on page 2. A JTS-150 sequence was developed to provide all of the in- formation seen by a PAM type fluorometer. All of the values required to calculate the fluorescence parameters is contained in the sequence above and without the introduction of as much potential actinic artifact that may be caused by the measuring beam of a PAM throughout the experiment.